Technical Report1
TOP > Immunohistochemistry > Detection Systems > Technical Report1
Ⅰ.OBJECTIVE
Detection of three different antigens at different locations within the same tissue section
Ⅱ.SPECIMENS
20% buffered formalin-fixed, paraffin-embedded tissue section
Ⅲ.TECHNICAL ADVICE (Staining orders)
1st Detection of Antigen:
Detection of small amount of antigen with BCIP/NBT (Blue)
2nd Detection of Antigen:
Detection of cytoplasmic antigen or large amount of antigen with New Fuchsin (Red)
2nd Detection of Antigen:
Detection of nuclear antigen or moderate to large amount of antigen with DAB (Brown)
Ⅳ.STAINING PROCEDURES
01.Deparaffinization and Rehydration
01-1. Immerse the slide in xylene at RT 3 times for 3 min each.
01-2. Immerse the slide in 100% ethanol at RT 2 times for 3 min each.
01-3. Immerse the slide in 95% ethanol at RT 2 times for 3 min each.
01-4. Rinse the slide in PBS at RT for 5 min.
1st Detection of Antigen
02.Antigen Retrieval depend on the 1st primary antibody
02-1. Check the specification sheets of the 1st primary antibodies and if necessary, conduct the antigen retrieval with specific buffer, temperature and incubation time on the specification sheets.
02-2. Allow the slide to cool down at RT for 20 – 60 min.
The slide should be cooled down slowly.
Rinse the slide in PBS at RT 3 times for 5 min each.
03.Protein Blocking
Apply 10% Goat normal serum at RT for 10 min.
04.Add 1st Primary Antibody
04-1. Apply 1st primary antibody at 37°C for 1 hour.
04-2. Rinse the slide in PBS at RT 3 times for 5 min each.
05.Add  -Histofine® Simple Stain AP (M)
-Histofine® Simple Stain AP (M)
05-1. Apply  -Histofine® Simple Stain AP (M) at RT for 30 min.
-Histofine® Simple Stain AP (M) at RT for 30 min.
05-2. Rinse the slide in PBS at RT 3 times for 5 min each.
05-3. Rinse the slide in TBS at RT for 5 min.
06.Add BCIP/NBT substrate
06-1. Apply BCIP/NBT substrate solution.
Adjust the incubation time by microscopic observation.
06-2. Wash the slide with distilled water at RT for 5 min.
2nd Detection of Antigen
07.Inactivation treatment of the primary antibody and the enzyme conjugated polymer in the steps of the 1st Detection of Antigen based on the specification sheets of the 2nd primary antibody
07-1. Conduct Method-A or Method-B depend on the 2nd primary antibody
Method-A for the 2nd primary antibody NO Antigen Retrieval required
Fill the heat-resistant plastic staining jar with 10 mM Sodium citrate buffer at pH 6.0 and heat to 95°C.
Immerse the slide in the jar at 95°C for 10 min.
Method-B for the 2nd primary antibody Antigen Retrieval required
Fill the heat-resistant plastic staining jar with the specific buffer instructed by the specification sheets of the 2nd primary antibody and heat to 95°C.
Immerse the slide in the jar at 95°C for 40 min.
Allow the slide to cool down at RT for 20-60 min.
The slide should be cooled down slowly.
07-2. Rinse the slide in PBS at RT 3 times for 5 min each.
08.Protein Blocking
Apply 10% Goat normal serum at RT for 10 min.
09.Add 2nd Primary Antibody
09-1. Apply 2nd primary antibody at 37°C for 1 hour.
09-2. Rinse the slide in PBS at RT 3 times for 5 min each.
10.Add  -Histofine® Simple Stain AP (M)
-Histofine® Simple Stain AP (M)
10-1. Apply  -Histofine® Simple Stain AP (M) at RT for 30 min.
-Histofine® Simple Stain AP (M) at RT for 30 min.
10-2. Rinse the slide in PBS at RT 3 times for 5 min each.
10-3. Rinse the slide in TBS at RT for 5 min.
11.Add New Fuchsin substrate
11-1. Apply New Fuchsin substrate solution.
Adjust the reaction time by microscopic observation.
11-2. Wash the slide with distilled water at RT for 5 min.
3rd Detection of Antigen
12.Inactivation treatment of the primary antibody and the enzyme conjugated polymer in the steps of the 2nd Detection of Antigen based on the specification sheets of the 3rd primary antibody
12-1. Conduct method-A or method-B depend on the 3rd primary antibody
Method-A for the 3rd primary antibody NO Antigen Retrieval required
Fill the heat-resistant plastic staining jar with 10 mM Sodium citrate buffer at pH 6.0 and heat to 95°C.
Immerse the slide in the jar at 95°C for 10 min.
Method-B for the 3rd primary antibody Antigen Retrieval required
Fill the heat-resistant plastic staining jar with the specific buffer instructed by the specification sheets of the 3rd primary antibody and heat to 95°C.
Immerse the slide in the jar at 95°Cfor 40 min.
Allow the slide to cool down at RT for 20-60 min.
The slide should be cooled down slowly.
12-2. Rinse the slide in PBS at RT 3 times for 5 min each.
13.Quenching of endogenous peroxidase
13-1. Immerse the slide in 3% H2O2solution in absolute methanol at RT for 10 min.
13-2. Rinse the slide in PBS at RT 3 times for 5 min each.
14.Protein Bloking
Apply 10% Goat normal serum at RT for 10 min.
15.Add 3rd Primary Antibody
15-1. Apply 3rd primary antibody at 37°C for 1 hour.
15-2. Rinse the slide in PBS at RT 3 times for 5 min each.
16.Add  -Histofine® Simple Stain MAX PO (M)
-Histofine® Simple Stain MAX PO (M)
16-1. Apply  -Histofine® Simple Stain MAX PO (M) at RT for 30 min.
-Histofine® Simple Stain MAX PO (M) at RT for 30 min.
16-2. Rinse the slide in PBS at RT 3 times for 5 min each.
17.Add DAB substrate
17-1. Apply DAB substrate solution.
Adjust the reaction time by microscopic observation.
17-2. Wash the slide with distilled water at RT for 5 min.
18.Mounting
During the slide are wet by water, put one drop of a water-soluble mounting media and fix with the cover slip.
Ⅴ.STAINING RESULTS
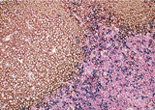
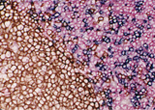
Case 1:Human Reactive Lymph Node
1.OBJECTIVE
Observe three types of stained cells in a tissue section.
2.SPECIMENS
Human Reactive Lymph Node
3.Used primary antibodies, antigen retrievals, detections and chromogens
1st Detection of Antigen:
| Primary Antibody: | CD8 |
|---|---|
| Antigen Retrieval: | 1mM buffered EDTA at pH8.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain AP (M) -Histofine® Simple Stain AP (M) |
|---|---|
| Chromogen: | BCIP/NBT (Blue) |
2nd Detection of Antigen:
| Primary Antibody: | CD4 |
|---|---|
| Antigen Retrieval: | 1mM buffered EDTA at pH8.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain AP (M) -Histofine® Simple Stain AP (M) |
|---|---|
| Chromogen: | New Fuchsin (Red) |
3rd Detection of Antigen:
| Primary Antibody: | CD20cy |
|---|---|
| Antigen Retrieval: | 10mM Sodium citrate buffer at pH 6.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain MAX PO (M) -Histofine® Simple Stain MAX PO (M) |
|---|---|
| Chromogen: | DAB (Brown) |
4.Photos
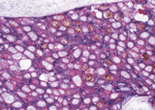
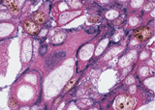
Case 2:Cervical Squamous Cell Carcinoma
1.OBJECTIVE
Observe three types of stained cells in a tissue section.
2.SPECIMENS
Cervical Squamous Cell Carcinoma
3.Used primary antibodies, antigen retrievals, detections and chromogens
1st Detection of Antigen:
| Primary Antibody: | Beta-catenin |
|---|---|
| Antigen Retrieval: | 1mM buffered EDTA at pH8.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain AP (M) -Histofine® Simple Stain AP (M) |
|---|---|
| Chromogen: | BCIP/NBT (Blue) |
2nd Detection of Antigen:
| Primary Antibody: | Cytokeratin (AE1/AE3) |
|---|---|
| Antigen Retrieval: | 10mM Sodium citrate buffer at pH 6.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain AP (M) -Histofine® Simple Stain AP (M) |
|---|---|
| Chromogen: | New Fuchsin (Red) |
3rd Detection of Antigen:
| Primary Antibody: | Ki-67 antigen |
|---|---|
| Antigen Retrieval: | 10mM Sodium citrate buffer at pH6.0, 95°C for 40 min. |
| Detection: |  -Histofine® Simple Stain MAX PO (M) -Histofine® Simple Stain MAX PO (M) |
|---|---|
| Chromogen: | DAB (Brown) |
4.Photos
Ⅵ.TIPS FOR STAINING
Ⅵ-1.Advice on the staining schedule
Two-day separated completion of all the steps of the IHC triple-staining is available under the following conditions.
1st day
The reaction condition of 1st primary antibody should be at 4°C for over night.
2nd day
The reaction condition of both 2nd and 3rd primary antibodies should be at 37°C for 1 hour.
Ⅵ-2.Chromogens Preparations
1.BCIP/NBT substrate solution
01-1. Reagents preparation
Substrate buffer (store at 2-8°C):
100 mM Tris-HCl Buffer (100 mM sodium chloride, 50mM MgCl2), pH 9.5
Adjust pH with HCl.
NBT stock solution (store at -20°C):
Dissolve 75 mg of NBT (Nitro Blue Tetrazolium, SIGMA) in 1 ml of 70% N,N-dimethylformamid.
BCIP stock solution (store at -20°C):
Dissolve 50 mg of BCIP (5-Bromo-4-Chloro-3-Indolyl Phosphate-p-Toluidine salt, SIGMA) in 1 ml of N,N-dimethylformamid.
01-2. Substrate solution preparation
Add 5 μl of BCIP stock solution and 6.5 μl of NBT stock solution to 1.5 ml of Substrate buffer and mix well.
Use the solution within 30 min after preparation.
2.New Fuchsin substrate solution
02-1. Reagents preparation
Naphthol AS-BI phosphate solution (Use within 30 min after preparation):
Dissolve 10 mg of naphthol AS-BI phosphoric acid (SIGMA) in 100 μl of N,N-dimethylformamid.
New fuchsin solution (store at 2-8°C):
Dissolve 4.0 g of New fuchsin powder (MERCK) in 100 ml of 2N HCl and filter the solution.
4% Sodium Nitrite solution (Use within 30 min after prepararion):
Dissolve 40 mg of Sodium Nitrite in 1ml of distilled water
0.2 M Tris-HCl buffer (store at room temperature):
200mM Tris-HCl buffer, pH 8.2-8.3
Adjust pH with HCl.
02-2. Substrate solution preparation
Mix 100 μl of New fuchsin solution and 100μl of 4 % Sodium Nitrite solution and incubate for 1 min.
Add 40ml of 0.2N Tris-HCl buffer to the mixture
Add 100 μl of Naphthol AS-BI phosphate solution to the mixture while stirring constantly.
Use the solution immediately after Filtration.
3.DAB substrate solution
Dissolve and mix following reagents and stir the solution.
Use the solution within 30 min after preparation.
10 mg of 3,3′-Diaminobenzidine,tetrahydrochloride
50 ml of 0.05 M Tris-HCl buffer, 15mM NaN3 pH 7.6
50 μl of 5% H2O2 in distilled water
34 mg of Imidazole